What Carries an Electric Current Through a Solution
When water is moving through a pipe the energy of the system is the potential energy of whatever is giving the water its pressure -- a column of water say -- and the kinetic energy of the water molecules as they move. Answer 1 of 2.
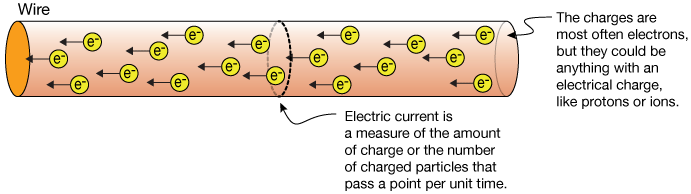
What is the direction of the electric current produced by an electron that falls toward the ground.

. A substance containing free ions which are the carriers of electric current in the electrolyte. What carries an electric current through a solution. Carries electric current well even at low solubility.
If the electron falls toward the ground. Up to 24 cash back Solution. Ions in solution is known as a _____ electrolyte.
A solution that breaks into its ions on passing electricity through it is called an electrolyte. D Electrorefining of metals. Solution The relation between drift velocity of electrons and current in a wire of cross-sectional area A is vd I ne A vd 003 x 10-3 m s-1 EXAMPLE 24.
It is the flow of IONs. Now the degree or the fraction of the current that is carried by the ion is known as the transport number or transference number. We can say that the current is caused So in this question the current is caused to tow the movement off.
The direction of conventional current is taken as the direction in which positive charge moves. Electricity is conducted through a solution containing an electrolyte by. Yields both ions and unionized molecules in solution.
Okay So basically the free irons carries an electric current through a solution. Then the direction of current is in the upward direction. The number of moles of a compound dissolved in one liter of a solution is called the _____.
A metal through which an electric current enters or leaves an electric cell for a specific electrolysis experimental set-up. A At the centre of the coil. The SI unit for current is the ampere A where 1 A 1 Cs.
A substance that carries an electric current when dissolved in water is called an _____. The current that passes through an electrolytic solution is carried via the ions. If the ions are not mobile as in a solid salt then electrolysis cannot occur.
By convention the direction of electric current is always in the opposite direction to the motion of negative charge. These ions then help in the conduction of electricity. An example of a nonelectrolyte is answer choices sugar water salt water sodium chloride hydrogen chloride Question 3.
Substances whose particles pass through filters but cannot pass through semipermeable. When you put salt in water the salt dissolved into solution. What is an electrolyte.
Obtain the magnitude of the magnetic field a at the centre of the loop and b at a distance of 97 cm from the centre of the loop but on the axis. When an electrical current is passed through a sodium chloride solution sodium hydroxide can be produced according to the following equation. This chemical reaction results in a release of energy through the flow of IONs.
The sodium and chloride ions actually separate in water turning solid NaCl into Na and Cl- ions that can move freely through the solution. If the free electron density of copper is 84 1028 m-3 then compute the drift velocity of free electrons. The ions that are present in a solution are the carriers of electrical current.
Electric current is carried by electrons in the external circuit. Electric current is often analogized to water under pressure in pipes. Water molecules pull the sodium and chlorine ions apart so they are floating freely.
These ions are what carry electricity through water as these charged particles can freely move. I ΔQ Δt I Δ Q Δ t where ΔQ Δ Q is the amount of charge passing through an area in time Δt Δ t. When a load is applied to the terminals it creates a closed circuit between the battery and load resulting in a chemical reaction.
R z 97 cm 97 10 -2 m I 23A N 1. Current in the power line I 90 A Point is located below the power line at distance r 15 m B 𝜇 0 2 x 𝐼 𝑟 4 𝑥 10 7𝑥 950 2 𝑥 15 12 x10-5 T The current is flowing from East to West. It uses a direct electric current DC to drive an otherwise non-spontaneous chemical reaction.
So Transport number of the cation n c. Provides the energy necessary to create or discharge the ions in the electrolyte. Electrons are one form of charge carriers and the most common being that they have a net negative charge and are mobile inside of metals but free ions moving around in a solution also constitutes a current.
And uh electrolytes are substances that dissociate in tow irons when dissolved in water so the. Saltwater like seawater on the other hand contains a lot of dissolved ionic compounds that split into ions in the solution. The chemist finds that they get 18 kg of sodium hydroxide.
The point is below the power line. Electrolysis is very important commercially as a stage in the separation of elements from naturally occurring sources such as ores. A copper wire of cross-sectional area 05 mm2 carries a current of 02 A.
A direct current DC supply. In this demonstration students will see how a solution can complete a circuit. 2NaCl H2O Cl2 H2 2NaOH A chemist carries out the above reaction using 40 kg of sodium chloride and 30 kg of water.
An electrode is a conductor of electricity that can carry electric current into non-metals and other poor conductors of electricity. The current is caused due to movement off electrons from one place to another. Electric current I is the rate at which charge flows given by.
Once characteristic of an anion is that. Therefore saltwater is a good conductor of electricity due to the presence of ions in the solution. Increase either their velocity by increasing the pressure or their total mass-per.
If an electrical current can run through a solution it is said to contain answer choices magic nonelectrolytes electricity electrolytes Question 2 30 seconds Q. These moving charges in. Electrolysis is a method of separating bonded elements and compounds by passing an electric current through them.
A circular loop of radius 97 cm carries a current 23 A. The phenomenon of decomposition of an electrolyte when electricity is passed through it is known as electrolysis. A solution that contains ions is called an electrolyte.
Electricity Electrons Vs Conventional Current

Electricity Understanding Electricity Electricity And Electric Currents
0 Response to "What Carries an Electric Current Through a Solution"
Post a Comment